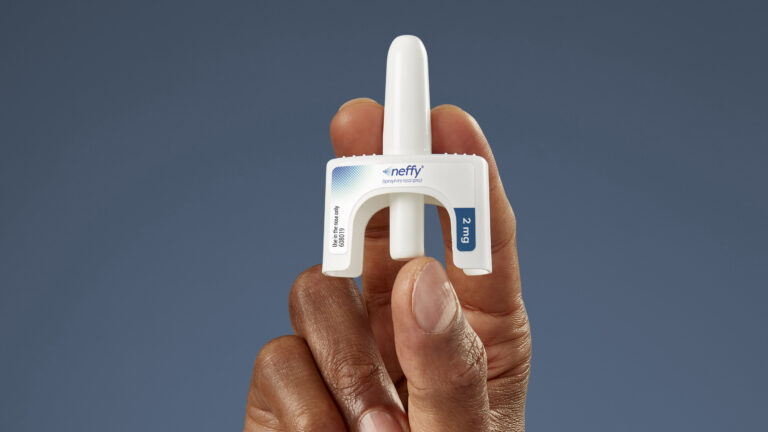
In a surprising decision, the FDA has chosen not to grant approval for a needle-free epinephrine nasal spray, which could have served as the first alternative to epinephrine autoinjectors like EpiPens.
Despite the FDA’s advisory committee recommending Neffy’s approval for both children and adults in May, the agency has requested additional research from drugmaker ARS Pharmaceuticals before reconsidering its decision. This situation is uncommon, as FDA committee recommendations are typically followed when approving drugs.
“We are very surprised by this action,” stated Richard Lowenthal, ARS Pharmaceuticals’ CEO, in response to the FDA’s decision. The company plans to challenge the FDA’s request for additional data.
Epinephrine has been a crucial treatment for anaphylaxis, a severe allergic reaction, in the U.S. since 1901. Anaphylaxis can occur rapidly after exposure to allergens like peanuts or cat dander and can be fatal without prompt treatment.
Currently, all available epinephrine treatments require injection, posing challenges for individuals with needle phobia. Dr. Zachary Rubin, an allergist at Oak Brook Allergists in Illinois, expressed astonishment at the FDA’s choice, noting the absence of needle-free alternatives, despite years of demand from patients.
One key concern during the FDA advisory committee meeting in May was the lack of clinical data, particularly the absence of studies involving individuals experiencing anaphylaxis. ARS Pharmaceuticals argued that their nasal spray was “comparable” to an EpiPen based on animal and non-anaphylactic human studies. Despite reservations, the panel voted 16-6 in favor of adult use and 17-5 in favor of pediatric use of the drug.
Dr. Maryann Amirshahi, a professor of emergency medicine at Georgetown University School of Medicine and a member of the advisory committee, raised concerns about the lack of data regarding patients with anaphylaxis, leading her to vote against the drug on both occasions.
Amirshahi mentioned that during the hearing, she empathized with parents who described the trauma of administering shots to their children. However, as a parent, panel member, pharmacologist, and emergency room physician with experience in anaphylaxis cases, her primary worry was the potential failure of a drug to effectively treat a life-threatening condition.
Conducting a randomized controlled trial for a drug designed for emergency treatment of life-threatening allergic reactions poses ethical dilemmas. To perform such a trial, individuals experiencing severe allergic reactions would either receive the treatment or a placebo, a situation deemed unethical by Dr. Zachary Rubin.
Amirshahi suggested that it might be feasible to study the drug in people experiencing anaphylaxis within an allergist’s office or emergency room setting, where a fail-safe treatment is readily available. She expressed hope that the FDA’s rejection would provide the necessary time to conduct thorough research to ensure the drug’s effectiveness.
Amirshahi pointed out that while she recognizes the drug’s potential, it lacks sufficient efficacy data at this stage.
The FDA has requested an additional study comparing Neffy to an epinephrine autoinjector in individuals with allergen-induced nasal symptoms like sneezing, itching, and congestion. The drug manufacturer intends to resubmit its application to the FDA in the first half of 2024.
(Source: Berkeley Lovelace Jr. | Sara G. Miller | NBC News)