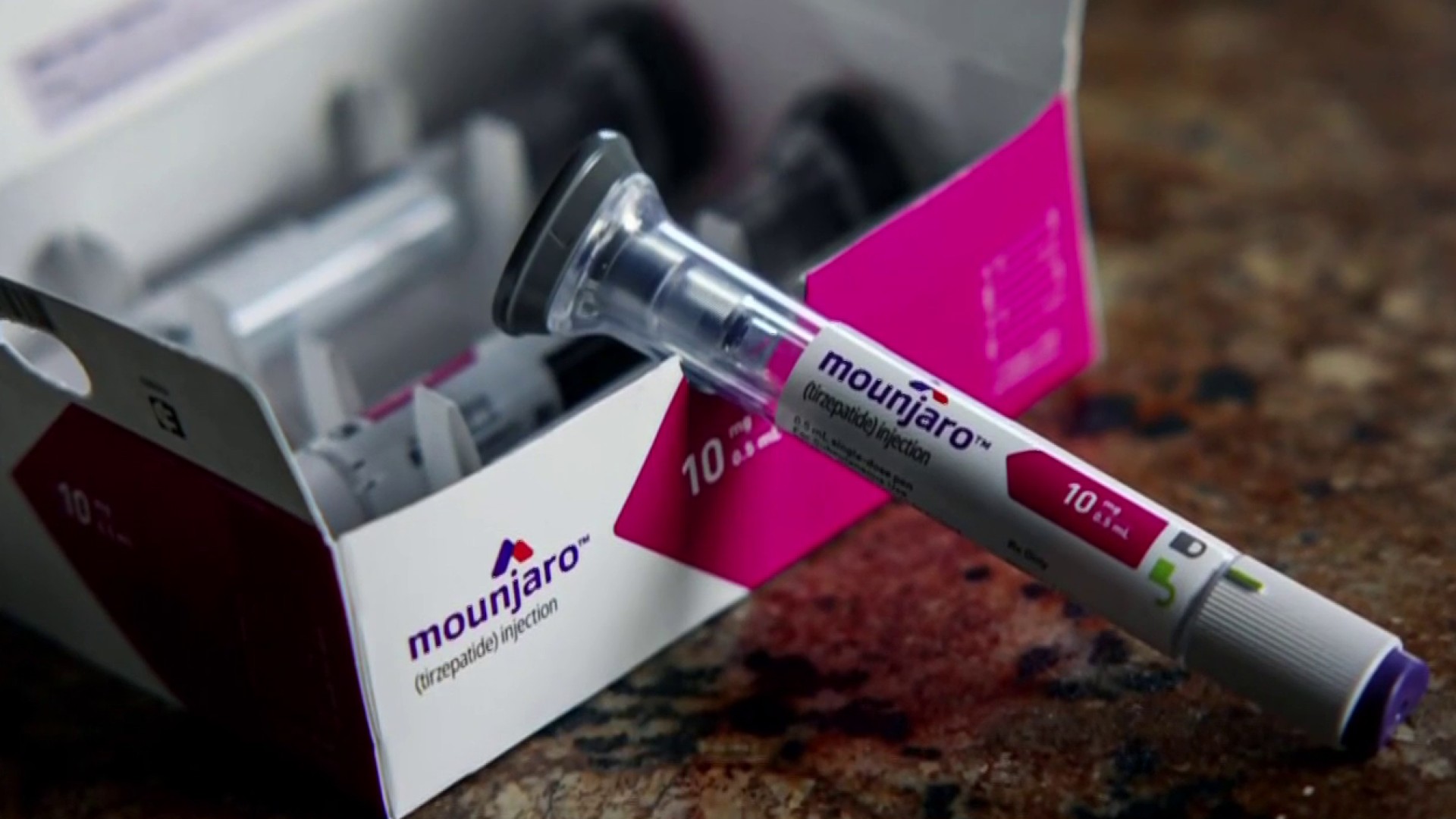
In a groundbreaking development, the Food and Drug Administration (FDA) has granted approval to Eli Lilly’s blockbuster drug, tirzepatide, for the treatment of obesity in adults. This decision paves the way for wider use of the medication, raising hopes for individuals seeking effective solutions to combat obesity. Tirzepatide, known for its efficacy in treating Type 2 diabetes under the name Mounjaro since May 2022, has emerged as a promising option for those struggling with obesity.
This approval comes amid a surge in demand for treatments that can facilitate significant weight loss, a trend accelerated by the success of Novo Nordisk’s Wegovy and Ozempic. All three medications, with list prices averaging around $1,000 per month, have been grappling with supply constraints due to their skyrocketing demand. The release of tirzepatide into the obesity treatment market reinforces Eli Lilly’s position as a formidable competitor to Novo Nordisk, setting the stage for an industry that Wall Street analysts believe could reach a staggering $100 billion by 2030. While the increased use of these drugs raises questions about their impact on various industries, it is still too early to determine how many individuals will choose this path for obesity management.
The FDA’s decision to approve tirzepatide for obesity is especially significant given the prevalence of this health issue. Obesity affects an estimated 650 million adults worldwide and approximately 40% of the adult population in the United States. The approval of this medication provides hope to those grappling with obesity, offering a potentially effective treatment option where others may have fallen short.
So, how does tirzepatide work its magic? This medication operates by activating two naturally produced hormones in the body: glucagon-like peptide-1, commonly referred to as GLP-1, and glucose-dependent insulinotropic polypeptide, known as GIP. The synergy between these two hormones slows down the emptying of the stomach, extending the feeling of fullness and curbing appetite by reducing hunger signals in the brain. This unique mechanism of action makes tirzepatide a promising contender in the battle against obesity.
As the world grapples with the implications of this FDA approval, it is essential to recognize the potential that tirzepatide and similar medications hold for those in need. While the road ahead may be long, this milestone opens new avenues for individuals seeking effective solutions to combat obesity, potentially changing lives and reshaping the landscape of the healthcare industry.
(Source: Annika Kim Constantino | CNBC | Madison Muller | Bloomberg)