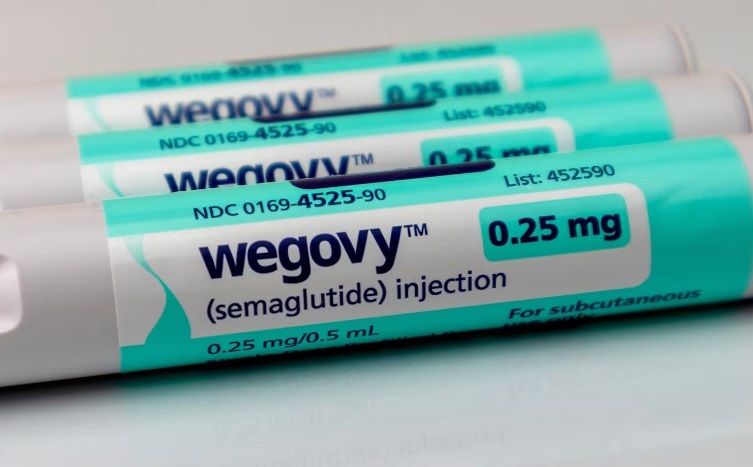
Novo Nordisk’s weight-loss drug Wegovy has received the green light in China, targeting the world’s second-largest economy and the country with the highest number of overweight or obese individuals. This development marks a significant milestone for the Denmark-based pharmaceutical giant as it enters a burgeoning market.
Novo Nordisk has yet to disclose details on Wegovy’s pricing and availability. The company announced that it would initially focus on Chinese patients willing to pay out-of-pocket for the weekly injectable drug.
China’s obesity epidemic is a pressing public health issue. By 2030, the number of overweight adults in China is projected to reach 540 million, a 2.8-fold increase from 2000. The number of obese adults is expected to rise to 150 million, a staggering 7.5-fold jump.
Novo Nordisk’s shares climbed 1.5% on the approval news, reaching record highs and valuing the company at nearly $490 billion. This uptick reflects investor optimism about Wegovy’s potential in China, despite looming challenges.
Novo Nordisk faces a shorter patent window for semaglutide, the key ingredient in Wegovy, in China. The patent is set to expire in less than two years, significantly earlier than in Europe, Japan, and the United States. Local competitors are swiftly developing generic and biosimilar versions, which could erode Novo Nordisk’s market share. Additionally, the company is embroiled in a legal battle over the patent, which could accelerate the loss of exclusivity.
Wegovy’s success has led to supply shortages, compelling Novo Nordisk to limit patient access. Capacity, not global demand, is the main limit on how fast sales volumes can grow. The addition of the Chinese market will further strain production capacity.
Novo Nordisk faces stiff competition from Eli Lilly, whose diabetes drug tirzepatide received Chinese approval in May. Eli Lilly’s weight-loss drug Zepound, featuring the same active ingredient, is anticipated to gain approval in China by 2025.
The weight-loss market is expected to exceed $100 billion globally by the decade’s end. Novo Nordisk and Eli Lilly are both investing heavily to scale up production. Novo Nordisk recently announced a $4.1 billion investment in a new U.S. facility to produce injection pens for Wegovy and Ozempic.
In China, local firms like Livzon Pharmaceutical Group and Hangzhou Jiuyuan Gene Engineering are gearing up to commercialize Ozempic copies. This domestic competition could further impact Novo Nordisk’s market share.
(Source: Market Watch | SCMP | Reuters)